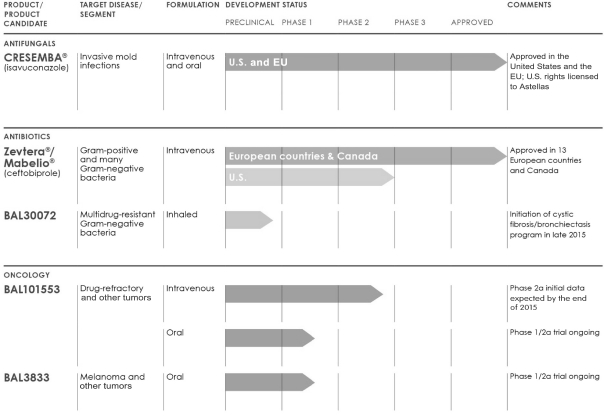
Mycamine Patent Expiration
expiration mycamine patent wallpaperPatent and Trademark Office at any time during a drugs development and may include a wide range of claims. Roche is forecasting a classic erosion of its injectable antibiotic Rocephin ceftriaxone after patent protection expired July 19 You may also be interested in.
Http Patentdocs Typepad Com Files Astellas V Fresenius 1 Pdf
Patent Number Expiration Date 6774104 the 104 patent January 8 2021 Your ANDA contains a paragraph IV certification to the 104 patent under section 505j2AviiIV of the FDC Act.
Mycamine patent expiration. Mycamine is a brand name of micafungin approved by the FDA in the following formulations. Jun 27 2006 Start Trial Start Trial. Additional patent term may also be privately granted by an Act of Congress and court decisions may establish patent expiration dates that are not reflected in USPTO records.
Providing detailed information on Brand Name Drug Patent Expiration since 2006 and on Medicare Part D plans for every state including selected Medicare Part D plan features and costs organized by State. Drug Patent Status Review - Patent Expiries Supplementary Protection Certificates Paediatric Extensions The drug patent status review database covers major product preparation patents together with any related supplementary protection certificates SPCs and paediatric extensions for all products on the European market including all 27 EU countries plus Switzerland. Mar 16 2005 Start Trial Start Trial.
Similarly19 patents of 7 drugs and 11 patents of 4 drugs are expiring in 2021 and 2022 respectively. _____ For questions on patent term USPTOs Office of Patent Legal Administration help line at 571-272-7702 is available as a resource. Patent and Trademark Office at any time during a drugs development and may include a wide range of claims.
April 16 2038 Patent use. Patent expiries Patent Expiry Definitions 29 October 2020 Year The year in which the patent expires this will be either the UK patent expiry or the UK SPC expiry if later. There are two patents protecting this drug and one Paragraph IV challenge.
If this can be avoided the product may face competition from generics. Patents are granted by the US. Micafungin sodium is the generic ingredient in two branded drugs marketed by Apotex Fresenius Kabi Usa and Astellas and is included in three NDAsThere is one patent protecting this compound and one Paragraph IV challenge.
TREATMENT OF PATIENTS WITH CANDIDEMIA ACUTE DISSEMINATED CANDIDIASIS CANDIDA PERITONITIS AND ABCESSES. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed Active Application number ES127139988T Other languages English en Other versions ES2547827T3. 2010-2020 PATENT EXPIRY DATES 13 The global generic pharmaceuticals market is likely to witness strong growth in the next few years owing to the patent expiration of key blockbuster drugs and the judicious cost containment efforts of governments and healthcare service providers worldwide.
Additional information is available in the individual branded drug. With its patent expired on January 5 only one patent will remain until its expiration in March 2025. Jun 27 2006 Start Trial Start Trial.
Patent Expiration Generic Entry Opportunity August 15 2021 Generic Entry Controlled by. Is a drug marketed by Ferring Pharms Inc. In 2020 25 patents of 11 drug are going to expire and lose protection and exclusitivity.
December 20 2022 - TREATMENT OF CANDIDEMIA ACUTE DISSEMINATED CANDIDIASIS CANDIDA PERITONITIS AND ABSCESSES WITHOUT MENINGOENCEPHALITIS ANDOR OCULAR DISSEMINATION IN PEDIATRIC PATIENTS YOUNGER THAN 4 MONTHS OF AGE June 20 2023 - PEDIATRIC EXCLUSIVITY. What are the generic drug sources for micafungin sodium and what is the scope of freedom to operate. So there will be an increase in the competition in generic drug market.
One tentatively approved generic is ready to enter the market. JP5818974B2 JP2014509598A JP2014509598A JP5818974B2 JP 5818974 B2 JP5818974 B2 JP 5818974B2 JP 2014509598 A JP2014509598 A JP 2014509598A JP 2014509598 A JP2014509598 A JP 2014509598A JP 5818974 B2 JP5818974 B2 JP 5818974B2 Authority JP Japan Prior art keywords compound formula sodium weak base solution Prior art date 2011-05-12 Legal status The legal status is an assumption and is not a. Information Expiry Status of Health Canada Patents covering Mycamine.
Micafungin purification procedure Prior art date 2011-04-20 Legal status The legal status is an assumption and is not a legal conclusion. Patents are granted by the US. A METHOD FOR THE IMPROVEMENT OF NEUROLOGICAL OUTCOME BY REDUCING THE INCIDENCE AND SEVERITY OF ISCHEMIC DEFICITS IN ADULT PATIENTS WITH SUBARACHNOID HEMORRHAGE SAH FROM RUPTURED INTRACRANIAL BERRY ANEURYSMS.
At the same time the balance in terms of healthcare expenditure and. Sign-up for our free Medicare Part D Newsletter Use the Online Calculators FAQs or contact us through our Helpdesk -- Powered by Q1Group LLC.

News In Proteomics Research When Does The Orbitrap Patent Expire Mass Spectrometry Patent Research

Antifungal Drugs Market Size Share Global Industry Growth 2027

Pdf May Your Drug Price Be Evergreen
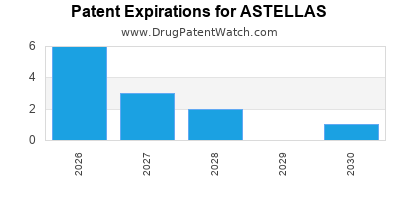
Astellas Branded And Generic Drugs International Patents
.jpg)
Drug Patent Expiry In 2021 Opportunities And Challenges In The Korean Market Managing Intellectual Property
Https Ir Cidara Com Static Files C4437ba1 1dc9 4b79 86ee 505b55114883
Mycamine Loss Of Exclusivity Loe When Do The Patents On Mycamine Expire And When Will Generic Mycamine Be Available

Pin On Medical Technology Infographics
Https Www Astellas Com En News 11026
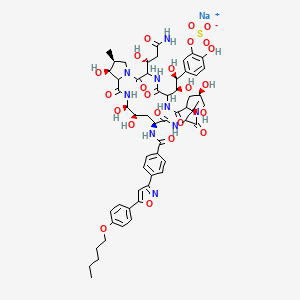
Mycamine C56h70n9nao23s Pubchem
Https Ec Europa Eu Transparency Regdoc Rep 10102 2018 En Swd 2018 240 F1 En Main Part 1 Pdf
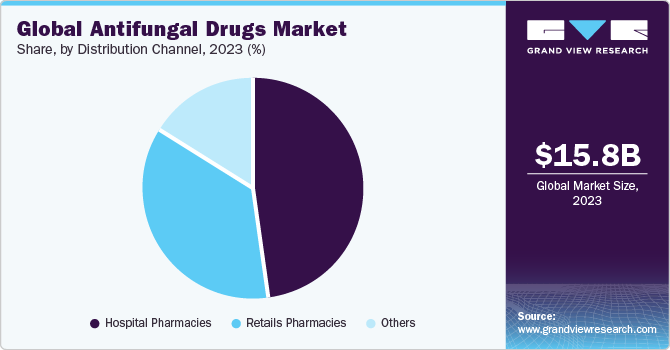
Antifungal Drugs Market Size Share Global Industry Report 2018 2025

Understanding Patents Logo Design Infographic Patent Agent Fun Facts